 Figure 1: DPSIR model - source: adapted from Smeets and Weterings, 1999
Figure 1: DPSIR model - source: adapted from Smeets and Weterings, 1999

| Address: | Dept. of Ecology, University of Palermo, Via delle Scienze, Ed. 16 I-90128 Palermo, Italy |
|---|---|
| Phone: | +39 091 6657932 |
| Email: | valeriapalmeri@unipa.it |
| C. Vitae: | |
| Specialization: | Ecology |
| Courses: |
Dynamic Energy Budget theory |
| Publications: | |
| Lectures: |
 A schematic view
A schematic view
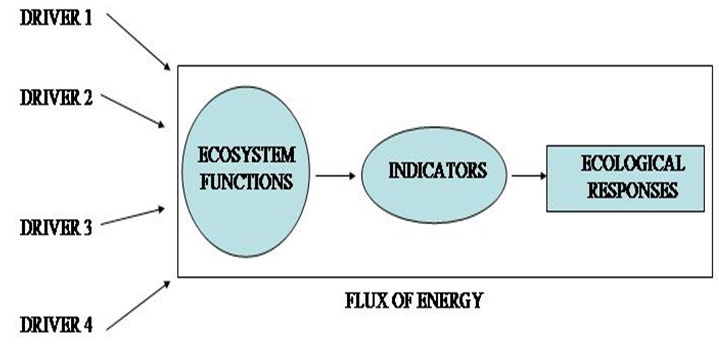
In this context, a reliable approach could be that offered by D.P.S.I.R. model (Driving forces, Pressures, States, Impacts, Responses) (see Figure 1) adopted by the OECD (Organisation for Economic Co-operation and Development 1996). That model allow us to assess how human activity exerts a pressure on the Environment and, as a main consequence, induces changes of the Environment's state. Unpredictable new states of the Environment may have impacts on people's health, ecosystem processes, structures and function and natural resources. These impacts can result in responses under the form of management approaches, policies, or actions that alter the driving forces, pressures, and, ultimately, the state of the Environment (European Environment Agency, 2003). Man, with all his activities (driving forces), causes stress (pressures) to the natural environment, whose conditions (state) tend to be modified as a consequence of this stress; wherever these modifications of environmental conditions turn out to be undesirable for man (impact), the anthropic system tends, in turn, to react (response) to the environmental change, to eliminate the causes or the consequences; in turn, when these responses are intended to eliminate the causes, they retroact more or less effectively on the pressures carried out by man on nature (OECD Workshop , 2003).
Primary driving forces are population growth and changes in people's
needs and activities. The importance of referring to an analytical
framework such as the DPSIR model could be useful to identify these
needs, which in turn exert pressures on the environment.
 Figure 1: DPSIR model - source: adapted from Smeets and Weterings, 1999
Figure 1: DPSIR model - source: adapted from Smeets and Weterings, 1999
The aim of this first chapter is to identify the principal drivers that can cause environmental change. A tool that can help us to do it could be the meta-analisysis. Meta-analytical reviewing technique offers major advantages for research synthesis in ecology of other review techniques (Gurevitch et al., 1992; Sarà 2007a, b, c). Throughout meta-analysis it is possible to focus the state of art, in the current literature, about the magnitude of a certain effect. The first step of a meta-analysis is to reduce the scope of the search, to the object of interest, using keywords such as: "driver - 1 (e.g high temperature) and environment and change", "driver - 2 (e.g. high pH) and impact", "driver - 3 (high pesticides) and ...". To obtain the general knowledge of the literature, is a way to provide a quantitative estimate of different drivers and to undertand what kind of effects they could generate on the Environment. This part will be assisted by Prof. Andrew Pullin and the staff of Centre for Evidence-Based Conservation University of Wales Bangor (UK).
De Groot (2002) in a influential recent paper pinpointed that across the ecological literature, the term "ecosystem function" has been used with various and sometimes contradictory interpretations. Sometimes the concept is used to describe the internal functioning of the ecosystem (e.g. maintenance of energy fluxes, nutrient (re)cycling, food-web interactions), and sometimes it relates to the benefits derived by humans from the properties and processes of ecosystems (e.g. food production and waste treatment). In general, we explicitly define ecosystem functions as "the capacity of natural processes and components to provide goods and services that satisfy human needs, directly or indirectly" (De Groot, 1992). However, a simple question could be: what is the role of each species to regulate ecosystem functions? And does species diversity (per se) affect the ecosystem functions?
Answering to these questions is not simple and we need to classify species as belonging to "trophic level" or "functional group". Such a classification has been suggested as a simplification of species effects on ecosystem properties, and the effects of global change on species interactions (Vitousek and Hooper, Chap 1 In: Biodiversity and ecosystem function, 1994). In particular, the present thesis's project proposes to consider intertidal organisms under a functional-trophic point of view. Thus, firstly, attention will be paid on one among the most important functional group of aquatic ecosystems, the filter feeders. Target environments will be represented by the intertidal and typical Mediterranean filter feeders are Mytilus galloprovincialis, Mytilaster minimus, Brachidontes pharaonis, Modiolus barbatus, Ostrea edulis, Dendropoma petraeum and Balanus spp.
Most of filter feeders like bivalves, barnacles and Dendropoma petraeum are among the most important ecosystem engineers in aquatic habitats (sensu Gutierrez et al. 2003). Their role in ecosystems is particularly attractive as tools to study global ecological processes; in fact, they are ubiquitous sessile animals in the Mediterranean like in all worldwide intertidal habitats; they are able to play a key-role in modifying and structuring worldwide intertidal/subtidal habitats (sensu Jones et al. 1994; 1997; Gutierrez et al 2003; Bayne 2004; Manganaro in press; Sousa et al. in press). As other ectothermic organisms, they are highly sensitive to environmental stress including increased warming (Menge et al. 2008), changes of pH (sensu Hall-Spencer et al 2008) and food availability induced by organic pollution (Sarà et al. submitted to Global Ecology and Biogeography) and chemical contaminants (Corsolini et al. 2007). They acquire energy from the available food and transform it into biological structures (e.g. shells) at a rate directly influenced by external temperature. Thus, in a context where coastal habitats changing is considered a global concern, it is important to ask whether and how human induced changes may affect stability/presence of ecosystem engineers.
In addition, it is well known that filter feeders play important role in the canalisation and distribution of organic matter, influencing ecosystem's functions. Filter feeders can have a top-down control on phytoplancton production, reducing the amount of available suspended organic carbon inducing some diastrophic processes like anoxia (Ward and Shumway, 2004). Filtration of particles reduces local turbidity, thereby increasing light availability to the bottom and enhancing the growth of benthic seagrasses (Newell and Ott, 1999; Sarà, 2006; Manganaro et al. in press). Therefore intertidal filter feeders may play the important role of ecosystem engineers affecting the functions of this ecosystem (Guitierrez et al., 2003).
Together filter feeders, in the intertidal there are many other important groups of key-organisms like periwinkles and limpets able to play important roles in the intertidal organic matter fluxes and general ecological equilibria. In this context, it is clear that energy flux through species, populations and communities affect structures and ecosystem functioning (Ernest et al. 2004; Duarte 2007). Energy assumes a central role as energy from food is allocated at a certain rate to the basal metabolic maintenance (i.e. minimum threshold required for persistence; respiration and excretion), growth, development and reproduction (Ernest et al. 2004) and that depends upon metabolism (Brown et al 2004). Metabolism depends upon temperature which controls and regulates biochemical kinetics (Brown et al. 2003; 2004; Clarke and Gaston 2006). For animals with indeterminate growth (animals growing rapidly when young for quickly reaching mature gonads sensu Charnov 1993) like most of marine invertebrates, is well known that the more the energy available for the organism, the more the somatic growth and the gonadal output. High quality and abundant gonadal tissue warrant abundant gametes which represent the condicio sine qua non to insure viable populations over time (Charnov 1993).
Consequently, we need a model linking the energy utilised for growth, reproduction and respiration of individual organisms to energy from available food may. The Dynamic Energy Budget model (DEB; sensu Koojiman 2000) could satisfy the most of these needs. Indeed, to describe the energy flow through individual organisms and consequently through populations and communities, Koojiman (2000) developed a model which resulted useful (Nisbet et al., 2000; Van der Veer et al., 2006; Bacher and Gangnery, 2006; Van der Meer 2006; Pouvreau et al., 2006; Ren and Schiel 2008; Alunno-Bruscia et al in press; Freitas et al in press) in assessing how the energy available from assimilated food is allocated to maintenance, growth, development and reproduction. An efficient approach to estimate the various DEB parameters is represented by experiments in which growth, reproduction and respiration are simultaneously estimated, preferably under varying feeding conditions and food intake (Kooijman, 1993, 2000). To do it, we use methodological approach commonly used in determining feeding rate (Van Haren and Kooijman, 1993), respiration (Van der Veer et al., 2006) and excretion rate (Van der Meer, 2006) as applied from the recent literature on bivalve Scope for Growth (SFG).
Under this context, it is likely that different drivers (e.g., temperature, pH, pollution etc.) may have different effects on the flux of energy to organism and, as a main consequence, on the ecosystem functions. K-rule DEB theory has been successfully applied in a variety of species such as: Crassostrea gigas (Pouvreau et al., 2006), Mytilus edulis, Cerastoderma edule (Van der Veer et al., 2006), etc. One of the purpose of this thesis is to estimate DEB parameter for the most key species of the Mediterranean intertidal habitats as: bivalves Mytilus galloprovincialis, Mytilaster minimus, Brachidontes pharaonis, Modiolus barbatus, Cellana rota, Ostrea edulis; gastropods Dendropoma petraeum, Melaraphe punctatus; limpets Patella sp.; barnacles Balanus spp. and lastly also some rock pool and sublittoral fish Gobius spp., Chromis chromis. We believe that, although these latter species are not truly intertidal filter feeders or primary consumers (the first is a detritivorous while the second is the most important sublittoral coastal zooplankter in the Mediterranean) they play an important role in intertidal habitats as the most of their functions have direct repercussions on intertidal habitats.
However, estimation of these parameters is complicated and can often
not be done in a direct way (Van der Meer, 2006). To combine
measurement such as clearance rates, feeding rates, respiration rates
and excretion rates it would be useful to asses the energy balance of
an individual, i.e. the energy potentially available for growth (rates
of energy acquisition: feeding and absorption and energy expenditure:
metabolism and excretion) (sensu Winberg 1960, Smaal & Widdows 1994;
Sarà and Pusceddu 2008). Therefore some of the potential energy is
converted by the reactions of photosynthesis and respiration into
biologically useful forms that are used to perform biological
processes such as metabolism. The metabolic rate is the fundamental
biological rate, because it is the rate of:
i) energy uptake;
ii) tranformation and
iii) allocation.
For a heterotroph, the metabolic rate is equal to the rate of
respiration because heterotrophs get energy by oxidizing carbon
compounds as described by the reaction:
CH2O + O2 -> energy + CO2 + H2O
(Brown et al., 2004).
Through the metabolism, organisms convert the resources take up from
the environment into energy. Consequently this energy was allocated
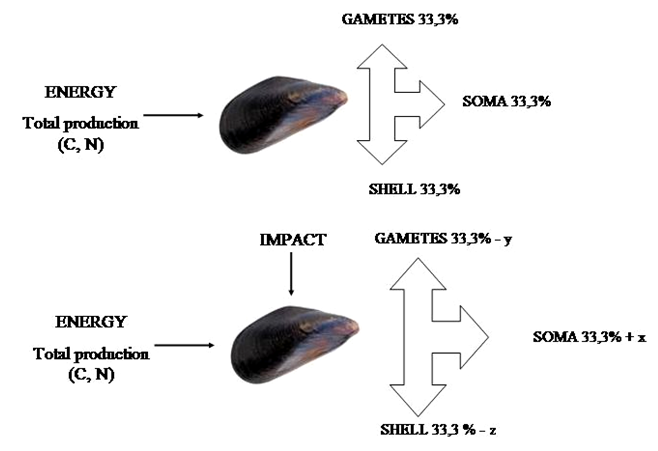
into different compartments. Some questions arise and one among the
most important is whether differences in metabolic rate or growth rate
between impacted and non impacted organisms may be explained by
differences in allocation of energy (carbon and nitrogen resources)
between somatic growth, shell growth and gametic output.
To understand how metabolic rate changes between organisms with different characteristics we can evaluate the effects of body size and temperature on individual metabolic rate. Gillooly et al. (2001) have proposed that this effect can be described by a single equation:
I = i0 M3/4e- E/kT,
where I = individual metabolic rate, M = body size, E = the activation energy of metabolism (defined as the average activation energy for the rate-limiting enzyme catalysed biochemical reactions of metabolism), T = absolute temperature, k = Boltzmann's constant and i0 = a normalization constant independent of M and T. Gillooly et al. (2001) called the temperature dependence term of this equation the Universal Temperature Dependence (UTD) of metabolism. The key assumption underlying the UTD is that metabolic rate is driven mechanistically by temperature. However, this assumpition is incompatible with what we know of cellular physiology and the molecular mechanisms of evolutionary adaptation to temperature (Clarke 2004).
Basal metabolism or resting metabolism is the key mechanism that
allows us to understand what the rate of energy required by an
organism to grow. Basal metabolism is defined as the matabolic rate of
an organism whose food intake is such that there is no net change in
body mass. Each higher food intake results in positive growth of body
mass, and this has been determined experimentally in many organisms by
measuring growth rate as a function of food intake. A pragmatic
alternative is to estimate basal metabolism by the oxygen consumption
of an inactive, non-growing and non reproducing individual. This is
often termed resting metabolism (Clarke and Fraser 2004). Although it
has long been known that in marine organisms basal metabolic rate (as
estimated by resting metabolic rate), increases with temperature, we
need to provide further information. One of the purpose of this
project is to evaluate if the increase of growth rate
(temperature-dependent), can determine a different allocation of
energy resource. There are numerous energetic models that explain the
growth of molluscs according to their environment, i.e. temperature
and food supply (Pouvreau et al., 2006). Some of these models are
based on the DEB model. This model also refers to the concepts of body
size and temperature's effects on metabolic rates, but it uses
terms such as surface areas, volumes and Arrhenius temperature (Figure
2). Variables such as size of shell, age and weight need to evaluate
the state of the organism's temperature and food dependencet.

Hence, the general aim of this thesis is to asses if this model is a
powerful tool to link the ecosystem function of ecosystem engineers to
resourse allocation of energy. In this way, we would be able to get
knowledge on the effect of the ecosystem changes causing direct impact
to population dynamics in terms of biodiversity losses.
