Predictions & Data for this entry
| Model: std | climate: MA, MB | migrate: Mo | phylum: |
| COMPLETE = 2.9 | ecozone: MC | food: biCi, piHa | class: |
| MRE = 0.125 | habitat: 0bTd, biMcp, biMr | gender: Dtmf | order: |
| SMSE = 0.033 | embryo: Tt | reprod: O | family: |
Zero-variate data
| Data | Observed | Predicted | (RE) | Unit | Description | Reference |
|---|---|---|---|---|---|---|
| ah | 47.69 | 47.34 | (0.007352) | d | age at hatch | StubMitc |
| ab | 54 | 49.43 | (0.08454) | d | age at birth | BalaRoss1974, Chri1990, StubMitc, Hend1958, PereBoot2011, SalmHama2009 |
| ap | 1.058e+04 | 6996 | (0.3391) | d | time since birth at puberty | BalaChal2004, BellPars2005, ChalLimp2004, FrazEhrh1985, FrazLadn1986, GoshAven2010, VanHHarg2014, ZuriHerr2012 |
| am | 2.92e+04 | 2.802e+04 | (0.0403) | d | life span | BalaChal2004, CSIRO |
| Lh | 4.55 | 4.562 | (0.002551) | cm | SCL at hatch | StubMitc |
| Lp | 91 | 97.87 | (0.07545) | cm | CCL at puberty | CSIRO |
| Li | 115 | 103.1 | (0.1039) | cm | ultimate CCL | Prin2017 |
| Li_ref | 143 | 128.8 | (0.09919) | cm | ultimate CCL | MoreBapt1995 |
| Wwh | 27.02 | 24.3 | (0.1005) | g | wet weight at hatch | StubMitc |
| Wwp | 9.7e+04 | 1.085e+05 | (0.1181) | g | wet weight at puberty | CSIRO |
| Wwi | 1.3e+05 | 1.266e+05 | (0.02591) | g | ultimate wet weight | CSIRO |
| E0 | 2.26e+05 | 2.166e+05 | (0.04157) | J | initial energy content of the egg | VenkKann2005, Wine2016, RuslBoot2016 |
| Ri | 0.3 | 0.2774 | (0.07536) | #/d | maximum reprod rate | BjorCarr1989, BrodGlen2003, EkanKapu2016, Guin2009, Limp1993, Limp2009, LimpMill2003, LimpNich1988, TroeChal2007 |
Uni- and bivariate data
| Data | Figure | Independent variable | Dependent variable | (RE) | Reference |
|---|---|---|---|---|---|
| tWwe_Y_T27B | 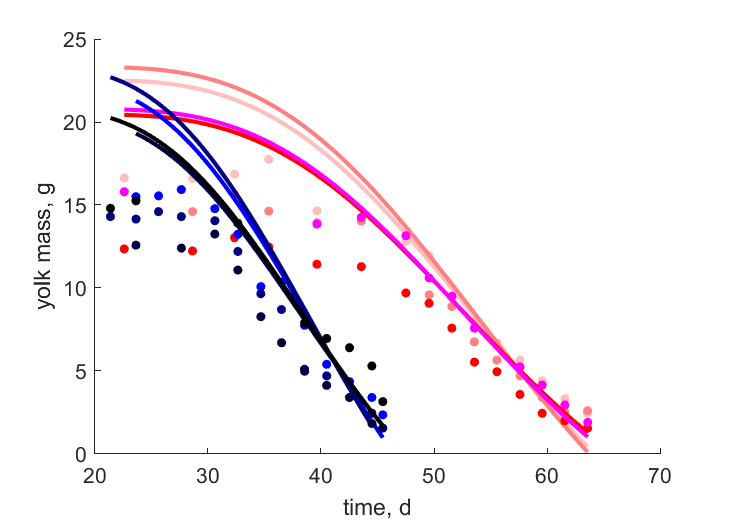  | time | yolk mass | (0.1942) | StubMitc |
| tWwe_Y_T27F |   | time | yolk mass | (0.3528) | StubMitc |
| tWwe_Y_T27H |   | time | yolk mass | (0.4207) | StubMitc |
| tWwe_Y_T27X |   | time | yolk mass | (0.1287) | StubMitc |
| tWwe_Y_T31B |   | time | yolk mass | (0.2211) | StubMitc |
| tWwe_Y_T31F |   | time | yolk mass | (0.4069) | StubMitc |
| tWwe_Y_T31H |   | time | yolk mass | (0.3801) | StubMitc |
| tWwe_Y_T31X |   | time | yolk mass | (0.2343) | StubMitc |
| tWwe_T27B | 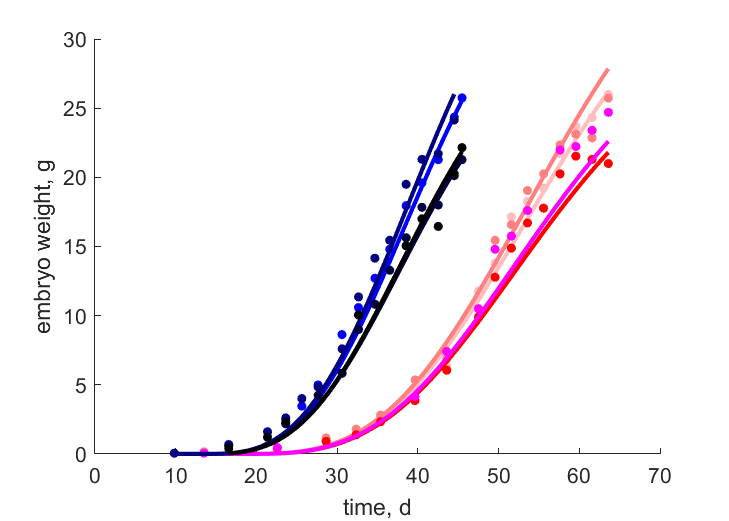  | time | embryo weight | (0.05177) | StubMitc |
| tWwe_T27F |   | time | embryo weight | (0.07197) | StubMitc |
| tWwe_T27H |   | time | embryo weight | (0.0973) | StubMitc |
| tWwe_T27X |   | time | embryo weight | (0.1252) | StubMitc |
| tWwe_T31B |   | time | embryo weight | (0.06978) | StubMitc |
| tWwe_T31F |   | time | embryo weight | (0.1056) | StubMitc |
| tWwe_T31H |   | time | embryo weight | (0.06839) | StubMitc |
| tWwe_T31X |   | time | embryo weight | (0.08029) | StubMitc |
| tJOe_T27B | 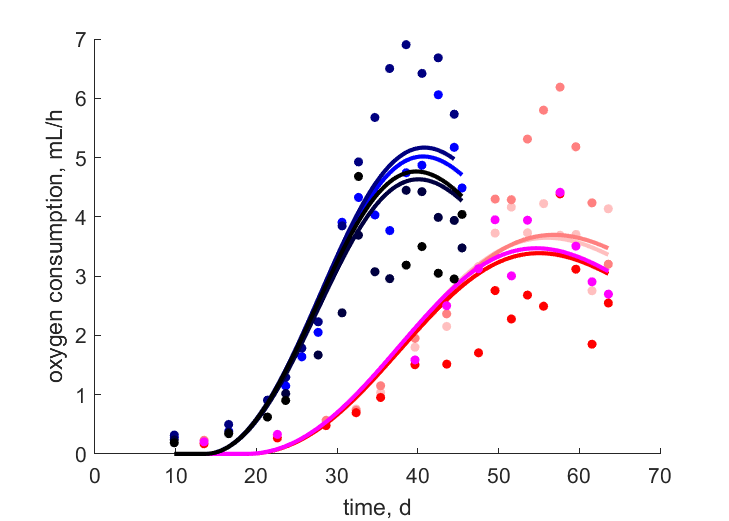  | time | oxygen consumption | (0.1323) | StubMitc |
| tJOe_T27F |   | time | oxygen consumption | (0.258) | StubMitc |
| tJOe_T27H |   | time | oxygen consumption | (0.3453) | StubMitc |
| tJOe_T27X |   | time | oxygen consumption | (0.143) | StubMitc |
| tJOe_T31B |   | time | oxygen consumption | (0.1242) | StubMitc |
| tJOe_T31F |   | time | oxygen consumption | (0.2006) | StubMitc |
| tJOe_T31H |   | time | oxygen consumption | (0.2043) | StubMitc |
| tJOe_T31X |   | time | oxygen consumption | (0.347) | StubMitc |
| tJCe_T27B | 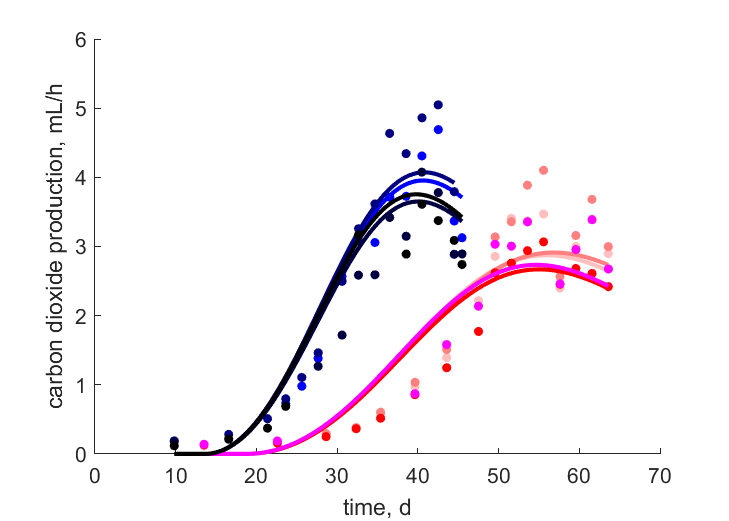  | time | carbon dioxide production | (0.2117) | StubMitc |
| tJCe_T27F |   | time | carbon dioxide production | (0.2395) | StubMitc |
| tJCe_T27H |   | time | carbon dioxide production | (0.1815) | StubMitc |
| tJCe_T27X |   | time | carbon dioxide production | (0.1929) | StubMitc |
| tJCe_T31B |   | time | carbon dioxide production | (0.1382) | StubMitc |
| tJCe_T31F |   | time | carbon dioxide production | (0.1469) | StubMitc |
| tJCe_T31H |   | time | carbon dioxide production | (0.1858) | StubMitc |
| tJCe_T31X |   | time | carbon dioxide production | (0.1689) | StubMitc |
| L0Lt | 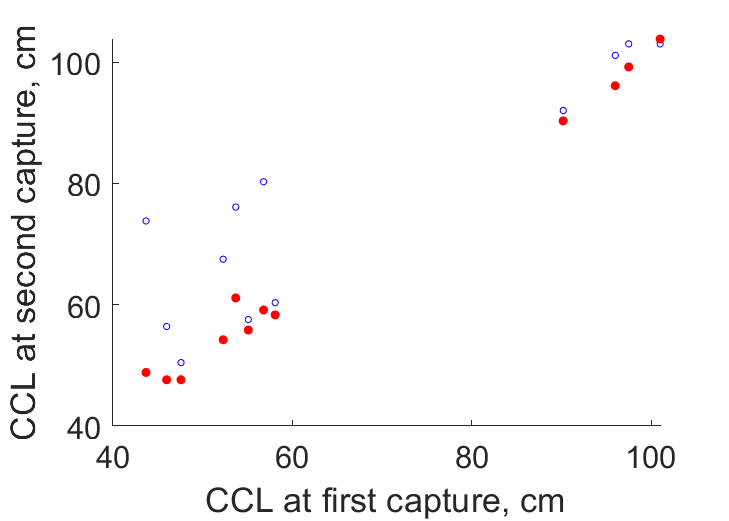 | CCL at first capture | CCL at second capture | (0.123) | CSIRO |
| LWw |  | CCL | weight | (0.06105) | CSIRO |
| Tah | 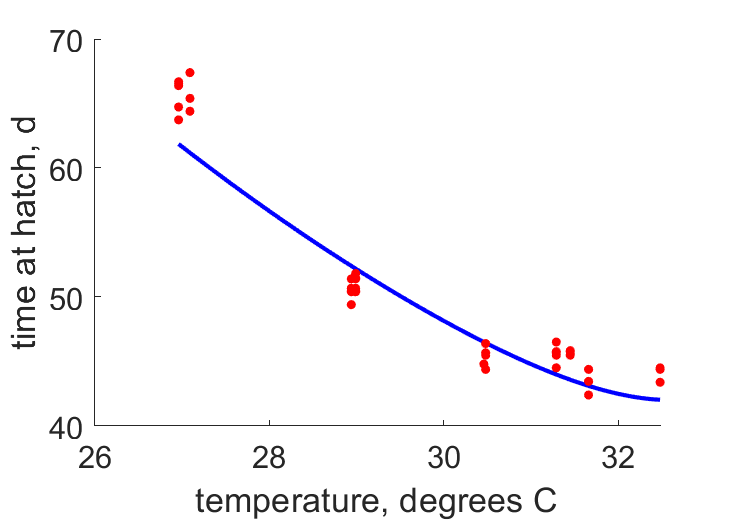 | temperature | time at hatch | (0.03947) | StubMitc |
Pseudo-data at Tref = 20°C
| Data | Generalised animal | Chelonia mydas | Unit | Description |
|---|---|---|---|---|
| v | 0.02 | 0.1057 | cm/d | energy conductance |
| kap | 0.8 | 0.7245 | - | allocation fraction to soma |
| kap_R | 0.95 | 0.95 | - | reproduction efficiency |
| p_M | 18 | 12.14 | J/d.cm^3 | vol-spec som maint |
| k_J | 0.002 | 0.001081 | 1/d | maturity maint rate coefficient |
| kap_G | 0.8 | 0.8 | - | growth efficiency |
| k | 0.75 | 0.6984 | - | maintenance ratio |
Discussion
- Mod_1: In view of low somatic maintenance, pseudodata k_J = 0.002 1/d is replaced by pseudodata k = 0.75
- Mod_2: data from the Ningaloo population are calculated for f=0.8 based on the ratio of the size attained by the Ningaloo population and the size of the largest published green turtle record
- Mod_2: we assume that the density of yolk is different than that of the density of reserve and structure. So the yolk density d_Y is introduced as an extra parameter.
- These parameter values and model predictions are presented and discussed in the paper: Stubbs et al, "A full life cycle Dynamic Energy Budget (DEB) model for the green sea turtle (Chelonia mydas) fitted to data on embryonic development"submitted to the Journal of Sea Research, DEB special issue 2018
- Mod_3: Mod_3: 3-parameter Arrhenius function describing upper boundary for thermal tolerance has been added to capture the slower rate of change of development rate at high temperatures more accurately. Additionally, pseudodata point k has been modified to k=0.75 to be more in line with other sea turtle models in the collection. See StubMitc2018 for a full discussion of this entry.
Bibliography