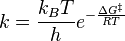Here, the energy changes during a chemical reaction are depicted:
A + B –> C + D
This reaction will proceed as indicated, because the energy level of the products is lower than that of the substrates ( i.e., ΔGo is negative ).
During most reactions, the energy change does not follow a linear path from substrates to products. Instead, reactants first have to gain energy in order to reach a higher energy level.
Enzymes stabilize the transition state by additional interactions. The released binding energy lowers the energy level and thereby accelerates the reaction.
Click through the links on this site or use the upper right icon to access controls for separate elements.