

Next: Bibliography
Up: exam
Previous: Key words
When a water molecule dissociates, one of its dipolar H-O bondsbreaks
into a positively charged hydrogen ion, H, and a negatively
charged hydroxyl ion, OH. The hydrogen ion, a proton,has a short
lifetime as a free particle; its combines with a water molecule to
form a hydronium ion, HO. This binding increases the
valence angle of the water molecule from to
, so making the added proton indistinguishable from the
other two. The extra proton possibly jumps from one water molecule to
another. The precise structure of liquid water is still not known with
certainty, but for practical purposes it is still convenient to refer
to the concentration of hydrogen ions in a solution, [H], even
though what is really meant is the concentration of hydronium ions.
The dissociation of water and the sociation of H and OH
are, in the mean in an otherwise constant environment, equally fast
processes and at C, we have
. In a pure water,
, which is frequently given in its
log-transformed form:
, where
is expressed as mole per litre.
The pH it one of the most important properties of a biological fluid.
It influences e.g. enzyme activities; a change in pH can trigger cell
growth and division; the movements of protons across a membrane, down
the electrochemical gradient, is coupled to the synthesis of ATP in
chloroplasts, mitochondria and bacteria. The pH is found to be
regulated within a narrow band around a pH of 7, rather independent of
the pH in the environment. Yet, there might be a lower limit to the
fluctuations due to the stochastic behaviour of the dissociation of
water. Think, for the sake of argument, of a bacterium of volume
, consisting of
pure water at C. For a specific density of
, it weights
g. The number of water
molecules is some
, while
the number of protons is
. The relatively small number of protons calls for a
consideration of the stochastic fluctuation of the number of 'free'
protons.
We assume that each water molecule dissociates independently
from the others with a constant probability rate of .
The probability rate of the binding of a proton with a hydroxyl
ion, is taken to be proportional to the product of their
concentrations (and so with their numbers in a fixed volume)
with a proportionality constant of . The number of protons,
say , is necessarily the same as the number of hydroxyl
ions because of electroneutrality and mass balance. We can safely
neglect the decrease of the number of water molecules, say 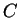 , due
to dissociation. If denotes the probability that the
number of protons at time
, due
to dissociation. If denotes the probability that the
number of protons at time 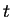 equals , we have for
equals , we have for
where refers to the probability that more than
one event (i.e. dissociation or binding) occurs during a
time increment . If we bring the term to the
left, divide by and let approach to zero,
we arrive at
where denotes the derivative of
with respect to the time. For we have
and so
which is a special case of (11.2) when we make the appointment
that . So, (11.2) represents the stochastic
model for the number of 'free' protons.
By comparison, the corresponding deterministic model would be
Separation of variables and integration gives
where denotes the equilibrium number of 'free' protons
in the deterministic model, which is given by
, and the relaxation time, which is
given by
. At C,
and
(in ice, is faster!).This gives a relaxation time of some s.
We now continue with a further analysis of the stochastic model. As
long as we are interested in processes with relaxation times much
longer than s, we can confine ourselves to the limiting
probability distribution of for large 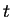 , where we have that
. When we divide by , call
,
as before, and abbreviate to , (11.2)
reduces in the limit to
, where we have that
. When we divide by , call
,
as before, and abbreviate to , (11.2)
reduces in the limit to
Starting with , and using (11.7) in the
form
we find by induction that
.
This relation determines the probabilities up to an arbitrary factor.
Obviously, we must have that
. The
series
is
well known as the modified Bessel function. So
. We therefore arrive at
This probability distribution relates to the Poisson
distribution by just squaring the Poisson probabilities and
renormalizing to assure that the sum of the probabilities remains
1. The normalizing constant in (11.8) compares
with in the Poisson distribution.
Since
, so
,
the expected number of protons equals
.
This is lower than the value , which should be expected on the
basis of the deterministic model. This is obvious when we obtain the
variance by summing (11.7) for
It is
found to be
. Although it is less
than the variance of a Poisson distribution with the same mean it is
still considerable for small , as is illustrated in Fig
11.1, which shows that the 95% confidence interval of the
pH in a
cell of neutral pH ranges from 6.85 to
7.15.
The lifetime of a randomly selected water molecule, a hydroxylion and
a hydronium ion follow an exponential distribution with mean
, i.e. some
h, for
water and , i.e. some s, for both ions at
C. Diffusion causes these particles to displace in time,
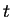 , over a mean distance of , where denotes the
diffusion coefficient. For HO, OH and H, the
latter is 2.26, 5.3 resp.
at C. The mean total lifetime displacement in an unbounded
body of pure water is thus 1.37cm, 3.26
, over a mean distance of , where denotes the
diffusion coefficient. For HO, OH and H, the
latter is 2.26, 5.3 resp.
at C. The mean total lifetime displacement in an unbounded
body of pure water is thus 1.37cm, 3.26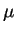 m and 4.32
m and 4.32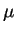 m, as a
crow flies. This means that the limited size of a cell is likely to
influence the transport, even apart from influences exercised by, e.g.
the membrane.
We made some simplifying assumptions. The first one is that
the cell consists of pure water, which is obviously not true. Its
cytoplasma is well buffered. Although a full analysis
would certainly be immensely complex, it is by no means certain
that the stochastic fluctuations are more restricted in buffered
mixtures. Buffers primarily balance net fluxes, but we did not
discuss that situation. The assumption that water molecules
dissociate independently from each other is hard to test at the
moment. The significance of the weak bonds certainly depends on
temperature. The intention of the example is to draw attention to
the odd behaviour of the concept proton 'concentration' in small
bodies. It might be relevant e.g. when we measure enzyme
performance in well mixed extracts and try to evaluate its
consequence for the living cell.
m, as a
crow flies. This means that the limited size of a cell is likely to
influence the transport, even apart from influences exercised by, e.g.
the membrane.
We made some simplifying assumptions. The first one is that
the cell consists of pure water, which is obviously not true. Its
cytoplasma is well buffered. Although a full analysis
would certainly be immensely complex, it is by no means certain
that the stochastic fluctuations are more restricted in buffered
mixtures. Buffers primarily balance net fluxes, but we did not
discuss that situation. The assumption that water molecules
dissociate independently from each other is hard to test at the
moment. The significance of the weak bonds certainly depends on
temperature. The intention of the example is to draw attention to
the odd behaviour of the concept proton 'concentration' in small
bodies. It might be relevant e.g. when we measure enzyme
performance in well mixed extracts and try to evaluate its
consequence for the living cell.


Next: Bibliography
Up: exam
Previous: Key words
Theoretische Biologie
2002-05-01