Analysis of routine biodegradation tests
Humankind has produced over 70.000 chemicals that are all sooner or
later released into the environment. In the sixties resistant
pesticides and persistent detergents caused environmental pollution.
Later the first tests on biodegradability were conceived and the
Environmental Protection Agency formed the Office of Pollution
Prevention and Toxics (OPPT) in 1977. Nowadays, new chemicals are
tested for toxicity and biodegradability before they are admitted to
the consumer market. These tests are carried out world-wide according
to the guidelines established by the Organization for Economic
Co-operation and Development, the European Union, the International
Organization for Standardization, and the Environmental Protection
Agency.
Task forces on biodegradation tests of, for example, the Society of
Toxicity and Environmental Chemistry (SETAC) and of the industry
recognize shortcomings in the protocols and in the interpretation of
standardized biodegradation tests, in particular with the analysis of
the test results.
Three areas have been selected to work on in this project:
- mass-transfer limitation and growth of microbial flocs
- multiple-substrate utilization and co-metabolism
- slow microbial adaptation to changing feeding conditions
These topics have been selected because "standard" models fail to
capture these important aspects of microbial degradation.
Flocculated growth
Microbes in activated sludge tanks mostly occur in flocs rather than
in cell suspensions.
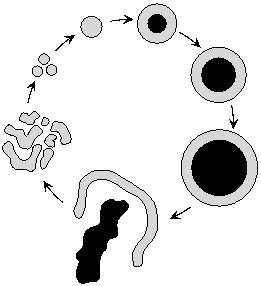 A microbial floc starts to grow with a diameter equal to the maximum
thickness of the living layer, develops a dead kernel (indicated in
black) and disintegrates. The biomass in the living layer is
redistributed to new flocs.
A microbial floc starts to grow with a diameter equal to the maximum
thickness of the living layer, develops a dead kernel (indicated in
black) and disintegrates. The biomass in the living layer is
redistributed to new flocs.
Flocculation results in a limited supply of substrate to the bacteria
inside the flocs, which reduces the biodegradation rate of organic
compounds by several orders of magnitude. A simple two-parameter
extension of growth models for cell suspensions was developed to
account for the ensuing reduction of the degradation rate. The
additional parameters represent floc size at division and diffusion
length. The biomass of small flocs initially increases exponentially
at a rate equal to that of cell suspensions. After this first phase,
the growth rate gradually decreases and finally the radius becomes a
linear function of time. At this time flocs are large and have a
kernel of dead biomass. This kernel arises when the substrate
concentration decreases below the threshold level at which cells are
just able to pay their maintenance costs. We deduced an explicit
approximative expression for the interdivision time of flocs, and
thereby for the growth of flocculated microbial biomass at constant
substrate concentrations. The model reveals that the effect of
stirring on degradation rates occurs through a reduction of the floc
size at division. The results can be applied in realistic
biodegradation quantifications in activated sludge tanks as long as
substrate concentrations change slowly.
Co-metabolism
The availability of multiple carbon/energy sources often enhances the
biodegradation of recalcitrant compounds. We classified and modelled
different modes of multiple substrate utilization in a systematic way,
using the concept Synthesizing Unit (generalized enzyme). According to
this concept, substrates can be substitutable or complementary; their
uptake (or processing) can be sequential or parallel. We show how the
different modes of multiple substrate interaction can be described by
a single general model. From the general model, we derive simple
expressions for co-metabolism of non structurally analogous
substrates. Both the general and the co-metabolism model have the
advantage that they can be used in combination with any microbial
growth model.
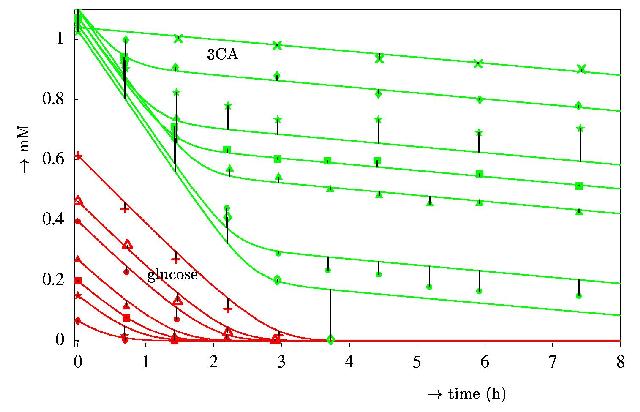
Result of model fit against data from Schukat et al 1983 on the
degradation of 3-chloroaniline (3CA) by Rhodococcus with
glucose as the primary substrate. Apart from a background
disappearence rate, 3CA is only degraded in the presence of glucose,
which is well-captured by the model. All 14 curves have been fitted
simulteneously.
The application of co-metabolism model to experimental data shows that
the general model constitutes a useful framework for modeling aspects
of multiple substrate utilization.
Adaptation
In their natural environment microorganisms encounter changes in
feeding conditions, involving either nutrient concentrations or
nutrient types. They have to adapt to the new conditions in order to
survive. We modelled the slow microbial adaptation in response to
changes in the availability of substrates. The model is based on
reciprocal (or mutual) inhibition of expression of both the
substrate-specific carriers and the associated assimilatory machinery.
The inhibition kinetics is derived from the kinetics of Synthesizing
Units. The model accounts for interaction among carriers by diffusion
limitation. The number of required adaptation parameters is one less
than the number of substrates that is involved. An interesting
property of the adaptation model is that the presence of a single
limiting resource results in a constant maximum specific
substrate consumption rate for fully adapted microorganisms. Because
the maximum specific consumption rate is not a function of substrate
concentration, for growth on one substrate, the Monod and Pirt models
for instance are still valid. Other adaptation models known to us do
not fulfil this property. The simplest version of our model describes
adaptation during diauxic growth, using only one preference parameter
and one initial condition. The applicability of the model is
exemplified by fitting it to published data from diauxic growth
experiments.

Model fit of growth of Pseudomonas oxalaticus (circles) on
oxalate (triangles) while adapting to acetate (blocks) on data by
Dijkhuizen et al 1980. The typical diauxic growth is well-captured by
the adaptation model. All three curves have been fitted
simultaneously.
This and other applications show the realism of the model.
Nederlandse samenvatting
Go to projects' entry
 A microbial floc starts to grow with a diameter equal to the maximum
thickness of the living layer, develops a dead kernel (indicated in
black) and disintegrates. The biomass in the living layer is
redistributed to new flocs.
A microbial floc starts to grow with a diameter equal to the maximum
thickness of the living layer, develops a dead kernel (indicated in
black) and disintegrates. The biomass in the living layer is
redistributed to new flocs.
